Respiration has a lot to do with the electron transport chain in cells. The electron transport chain is a number of protein complexes in the mitochondrial membrane. Two electrons are handed from NADH into the NADH dehydrogenase complex. Along with this transfer is the pumping of one hydrogen ion for each seperate electron. Secondly, the two electrons are transferred to ubiquinone(a mobile carrier because it moves the electrons to the cytochrome b-c1 complex). After that each electron is passed from the cytochrome b-c1 complex to cytochrome c. This only takes one electron at a time, pumping one hydrogen ion through the complex as each electron is transferred. Next, four electrons in the cytochrome oxidase complex interact with a molecular oxygen molecule and eight different hydrogen ions. The four electrons, only for of the hydrogen ions, and the molecular oxygen form two water molecules. The remaining four hydrogen ions are pumped across the membrane. Hydrogen pumping creates a gradient. The potential energy in the gradient is used up by ATP synthase to ATP from ADP and inorganic phosphate. Most electron transport cycles occur simultaneously to make sure the protein gradient is always maintained.
This really confused me at first, but after looking through various animations and having it explained to me about 999,999,999,999.876 times, I understand it better now.(:
Thursday, December 16, 2010
Photosynthesis
Photosynthesis is the process that creates ATP from ADP and Pi using light energy. The light energy "excites" the electrons passing along the electron transport chain. This is coupled with the pumping of hydrogen ions and the splitting of H2O molecules.
Photosynthesis occurs in the chloroplast of plant and algae cells. First, the light photon hits the chlorophyll molecule surrounding Photosystem II(complex). This creates a resonance energy that's transferred through neighboring chlorophyll molecules. When the energy reaches the Photosystem II reaction center, an electron is released. The chlorophyll reaction center contains electrons that are transferred when excited. Only one photon is needed to excite each electron in the chlorophyll.
Then, two excited electrons are transferred to plastoquinone Qb(the first mobile carrier). Along the way, the Qb also picks up two protons from the stroma. The two lost electrons are replaced by splitting H2O molecules. The splitting water also releases hydrogen ions into lumen. This contributes to hydrogen ion gradient like one created by the mitochondrial electron transport.
Qb will then transfer two electrons to cytochrome b6-f, allowing two protons to be released from the lumen. This is coupled with the pumping of two or more hydrogen ions into the lumen space by cytochrome b6-f. After this, electrons are transferred to plastocyanin and then Photosystem I. The photons energize the electrons and "propel" them to transfer to the ferredoxin. Ferredoxin transfers the electrons to FNR.
NADPH is made by adding 2 electrons and a hydrogen ion to NADP+. The result is NADPH, ATP, and molecular oxygen.
Photosynthesis occurs in the chloroplast of plant and algae cells. First, the light photon hits the chlorophyll molecule surrounding Photosystem II(complex). This creates a resonance energy that's transferred through neighboring chlorophyll molecules. When the energy reaches the Photosystem II reaction center, an electron is released. The chlorophyll reaction center contains electrons that are transferred when excited. Only one photon is needed to excite each electron in the chlorophyll.
Then, two excited electrons are transferred to plastoquinone Qb(the first mobile carrier). Along the way, the Qb also picks up two protons from the stroma. The two lost electrons are replaced by splitting H2O molecules. The splitting water also releases hydrogen ions into lumen. This contributes to hydrogen ion gradient like one created by the mitochondrial electron transport.
Qb will then transfer two electrons to cytochrome b6-f, allowing two protons to be released from the lumen. This is coupled with the pumping of two or more hydrogen ions into the lumen space by cytochrome b6-f. After this, electrons are transferred to plastocyanin and then Photosystem I. The photons energize the electrons and "propel" them to transfer to the ferredoxin. Ferredoxin transfers the electrons to FNR.
NADPH is made by adding 2 electrons and a hydrogen ion to NADP+. The result is NADPH, ATP, and molecular oxygen.
Tuesday, December 14, 2010
Saturday, December 11, 2010
Enzyme Lab
I never realized how easily a person can alter the pressure in a test tube with only a little water, H2O2, and yeast.
After mixing H2O2 and water in a test tube, we began our experiment by altering the number of yeast drops we put in the test tube.
The higher the number of yeast droplets, the higher the pressure kept climbing.
Next, we altered the temperature of the test tubes. We went back to mixing H2O2 with water in the test tube. Then, we left one at room temperature, one at 25 degrees, one at 38 degrees, and one at 80 degrees.
This graph is strange. The lowest temperature had the lowest pressure level. At 25 degrees the pressure level was slightly higher. Then, pressure spiked as it was at 38 degrees or warm. But, when the temperature was at 80 degrees, the pressure lowered slightly.
Lastly, instead of altering the number of yeast drops, we altered the pH level of the water concentration to determine how the pH level affects the pressure in the test tube.
The higher the pH levels, the higher the pressure. In two cases the cap actually popped off due to the increased pressure.
This lab taught me that little variable can affect pressure in a great way, making me wonder what else can affect pressure or other characteristics of chemicals...
After mixing H2O2 and water in a test tube, we began our experiment by altering the number of yeast drops we put in the test tube.
The higher the number of yeast droplets, the higher the pressure kept climbing.
Next, we altered the temperature of the test tubes. We went back to mixing H2O2 with water in the test tube. Then, we left one at room temperature, one at 25 degrees, one at 38 degrees, and one at 80 degrees.
This graph is strange. The lowest temperature had the lowest pressure level. At 25 degrees the pressure level was slightly higher. Then, pressure spiked as it was at 38 degrees or warm. But, when the temperature was at 80 degrees, the pressure lowered slightly.
Lastly, instead of altering the number of yeast drops, we altered the pH level of the water concentration to determine how the pH level affects the pressure in the test tube.
The higher the pH levels, the higher the pressure. In two cases the cap actually popped off due to the increased pressure.
This lab taught me that little variable can affect pressure in a great way, making me wonder what else can affect pressure or other characteristics of chemicals...
Friday, December 10, 2010
Photosynthesis Lab Write Up
Materials:
Bromothymol blue
2 aquarium snails
2 elodea (aquarium plant)
pond water
4 beakers
light lamp
Procedure:
1. Take all of the beakers and fill them 3/4 the way with pond water.
2. Take all of the beakers and mix bromothymol blue with the water until you see a color change. Record your results.
3. Take a beaker with bromothymol blue and water mixed in and place the snail into the beaker until you see a color change. Record your results.
4. Take the third beaker with bromothymol blue and water mixed in and place elodea in the beaker. Place the beaker under the lamp for three hours. Record the color change.
5. Take the last beaker with bromothymol blue and water mixed in, and place a snail and a elodea in the water. Leave in the light for three hours. Record the color change in the light.
6. Take the beaker from step 5 and place in it in the dark for three hours. Record the color change.
7. Clean up, return the snails to the wild, and DON'T DRINK THE POND WATER!
Observation/Conclusion:
1. Water plus bromothymol blue is blue green - because the water is a neutral pH.
2. Water plus bromothymol blue plus an aquarium snail turns yellow - because the snail produces carbon acid and BTB turns yellow in acid.
3. Water plus bromothymol blue plus elodea, is blue-green in light. -green plants photosynthesize in the light and respire all the time, but still keeping the pH neutral, allowing the water and BTB to remain blue-green.
4. Water plus bromothymol blue plus a snail plus elodea is blue-green in light and yellow when left in the dark for three hours. - because the carbon dioxide produced by the snail is soaked up by the elodea due to photosynthesis, allowing the water to remain blue-green in light. But, because in the dark, the elodea can't photosynthesize, the carbon dioxide from the snail turns the water acidic, making the water turn yellow when in the dark.
Bromothymol blue
2 aquarium snails
2 elodea (aquarium plant)
pond water
4 beakers
light lamp
Procedure:
1. Take all of the beakers and fill them 3/4 the way with pond water.
2. Take all of the beakers and mix bromothymol blue with the water until you see a color change. Record your results.
3. Take a beaker with bromothymol blue and water mixed in and place the snail into the beaker until you see a color change. Record your results.
4. Take the third beaker with bromothymol blue and water mixed in and place elodea in the beaker. Place the beaker under the lamp for three hours. Record the color change.
5. Take the last beaker with bromothymol blue and water mixed in, and place a snail and a elodea in the water. Leave in the light for three hours. Record the color change in the light.
6. Take the beaker from step 5 and place in it in the dark for three hours. Record the color change.
7. Clean up, return the snails to the wild, and DON'T DRINK THE POND WATER!
Observation/Conclusion:
1. Water plus bromothymol blue is blue green - because the water is a neutral pH.
2. Water plus bromothymol blue plus an aquarium snail turns yellow - because the snail produces carbon acid and BTB turns yellow in acid.
3. Water plus bromothymol blue plus elodea, is blue-green in light. -green plants photosynthesize in the light and respire all the time, but still keeping the pH neutral, allowing the water and BTB to remain blue-green.
4. Water plus bromothymol blue plus a snail plus elodea is blue-green in light and yellow when left in the dark for three hours. - because the carbon dioxide produced by the snail is soaked up by the elodea due to photosynthesis, allowing the water to remain blue-green in light. But, because in the dark, the elodea can't photosynthesize, the carbon dioxide from the snail turns the water acidic, making the water turn yellow when in the dark.
Thursday, December 2, 2010
Poison - Death Camas/Star Lily
Something that really surprised me was that a plant that looks relatively pretty, can be deadly? That's right! The Star Lily is a deadly plant! It is also known as the death camas. (Toxicoscordion fremontii (Torrey) Rydb in latin)The plant grows from a bulb, and can be mistaken for an onion. The Star Lily is most commonly found in the Western United States (us), some parts in the Eastern United States, and the North American, Western sub arctic and Eastern Siberia. All parts of the "death camas" are extremely toxic. All parts of this plant contain the poisonous alkaloid zygadenine Symptoms include: nervousness, frothing at the mouth, loss of muscle control, subnormal temperature, upset stomach, diarrhea, reduced heart rate, decreased blood pressure and respiration rate. A coma is possible.
The Star Lily is especially dangerous for cats and dogs if ingested, but humans have also been poisoned after eating the plant. Once, a 2 year-old-child became extremely ill after eating the plant. The alkaloids cause local irritation when ingested and affect the cardiovascular system by slowing the heart and decreasing blood pressure. Treatment includes emesis, activated charcoal, atropine and saline cathartic.
The Star Lily is especially dangerous for cats and dogs if ingested, but humans have also been poisoned after eating the plant. Once, a 2 year-old-child became extremely ill after eating the plant. The alkaloids cause local irritation when ingested and affect the cardiovascular system by slowing the heart and decreasing blood pressure. Treatment includes emesis, activated charcoal, atropine and saline cathartic.
http://helenair.com/lifestyles/recreation/article_0c268c4e-b7d8-5210-9a41-3da75cb76d9d.html
I think it's interesting that a plant can be poisonous. I never knew this before. Obviously in the jungle some plants aren't the best to eat, but a plant that can be right outside our door? That can poison our animals and children? That really surprised me. Luckily it's not extremely deadly towards children.
Friday, November 19, 2010
PKU (Phenylketonuria)
PAH is the enzyme that is most commonly defective in people with PKU.
PKU is hereditary and is carried as follows:
A person can accumulate dangerously high phenylalanine levels in the brain, poisoning the neurons, and causing mental retardation and epilepsy if not treated correctly.
A baby with PKU may have a smaller than normal head, epilepsy(seizures), and mental retardation. A musty odor may occur. Also, the deficiency in tyrosine leads to a lighter skin and hair color on the baby.
The lack of tyrosine and the buildup of phenylalanine levels cause the symptoms of PKU.
The commonality of PKU is as follows:
1 in 143,000 Japanese babies will be born with PKU.
1 in 10,000 Caucasian and East Asian babies will be born with PKU.
1 in 2,600 Turks babies will be born with PKU.
1 in 4,500 Irish babies will be born with PKU.
PKU can be treated by giving the baby a low protein diet for as long as possible. Even after they get out of the toddler and childhood years, a limited protein diet will help with the symptoms of PKU.
I've never known about this disease until now, and I think it's pretty interesting. I think testing newborn babies for this disease is wise so that the parents can learn quickly how to treat their children and allow them to grow up with a semi-normal life. This is sad, and hopefully some day we can come up with a quick cure for this disease so that less children will have to deal with it throughout their lifetimes.
PKU is hereditary and is carried as follows:
A person can accumulate dangerously high phenylalanine levels in the brain, poisoning the neurons, and causing mental retardation and epilepsy if not treated correctly.
A baby with PKU may have a smaller than normal head, epilepsy(seizures), and mental retardation. A musty odor may occur. Also, the deficiency in tyrosine leads to a lighter skin and hair color on the baby.
The lack of tyrosine and the buildup of phenylalanine levels cause the symptoms of PKU.
The commonality of PKU is as follows:
1 in 143,000 Japanese babies will be born with PKU.
1 in 10,000 Caucasian and East Asian babies will be born with PKU.
1 in 2,600 Turks babies will be born with PKU.
1 in 4,500 Irish babies will be born with PKU.
PKU can be treated by giving the baby a low protein diet for as long as possible. Even after they get out of the toddler and childhood years, a limited protein diet will help with the symptoms of PKU.
I've never known about this disease until now, and I think it's pretty interesting. I think testing newborn babies for this disease is wise so that the parents can learn quickly how to treat their children and allow them to grow up with a semi-normal life. This is sad, and hopefully some day we can come up with a quick cure for this disease so that less children will have to deal with it throughout their lifetimes.
Monday, November 15, 2010
Diffusion and Osmosis Lab!
Lab day woo! We started out by filling a cup with distilled water, then added about 4mL of IKI(iodine) and tested the brownish solution with an indicator strip, coming up with a green result meaning there is no glucose. Then we took a dialysis tube, tied one end of it, and filled it with about 15mL of 15% glucose/1% starch solution. The glucose/starch solution was clear in color but tested positive for glucose(obviously). We took the tube and tied the other end of it and placed it in the IKI solution.
I wasn't here for the second day, so I didn't get to see the results sadly...
This lab was a lot of fun because we got to feel like scientists(:
Microscopes!
Today!!!! ....and yesterday we played with the microscopes. We looked at pond water, foam, fruit loops, mandarin orange juice, plant leaves, and most exciting..... SOAP!!! Yes, that's right, soap! Hand-pumped, school soap! Here's a picture:
This activity was pretty cool because we got practice learning how to use the microscope like focusing it, playing with the magnification for each different substance, and learning not to put your eye up to the hole and then turning the light on (it can blind you.)
I really liked this activity because we got to use our imaginations on what to look at, but still got useful practice in using the microscopes.
This activity was pretty cool because we got practice learning how to use the microscope like focusing it, playing with the magnification for each different substance, and learning not to put your eye up to the hole and then turning the light on (it can blind you.)
I really liked this activity because we got to use our imaginations on what to look at, but still got useful practice in using the microscopes.
Tuesday, November 2, 2010
Our Poster!
 I was in a group with Gena, Kandace, and Shawn, where we made a poster of a diagram of a fluid mosaic membrane structure. This structure is particularly interesting. The structure has an outer layer of phospholipids, which are hydrophilic. The phospholipids surround the fatty acids, which are hydrophobic. Intertwined with the phospholipids and fatty acids, is a rather large proportionally transmembrane protein.The transmembrane protein is a protein that goes all the way across the structure. There is a glycoprotein. Coming off of the glycoprotein are carbohydrate side chains. And there is an integral protein. Put together in the right structure, you can form a fluid mosaic membrane structure. (Here is a not-so-great picture of our wonderful creation) ------>
I was in a group with Gena, Kandace, and Shawn, where we made a poster of a diagram of a fluid mosaic membrane structure. This structure is particularly interesting. The structure has an outer layer of phospholipids, which are hydrophilic. The phospholipids surround the fatty acids, which are hydrophobic. Intertwined with the phospholipids and fatty acids, is a rather large proportionally transmembrane protein.The transmembrane protein is a protein that goes all the way across the structure. There is a glycoprotein. Coming off of the glycoprotein are carbohydrate side chains. And there is an integral protein. Put together in the right structure, you can form a fluid mosaic membrane structure. (Here is a not-so-great picture of our wonderful creation) ------>
Thursday, October 7, 2010
Since I bombed my test...
For all of you who don't know, I hate taking tests, and obviously I didn't do so well on this last quiz that we had. So, I thought in order to prove that I know what I'm talking about, I'd do a blog post about everything I've gotten out of Biology about saccharides, glycogen, carbohydrates,etc. Great use of technology right?
A monosaccharide means one or single. It includes glucose, galactose, and fructose. Food examples of this includes honey, fruit, high-fructose corn syrup(which we get from fructose) and milk(from galactose).
A disaccharide means two or double. When a disaccharide is created, one monosaccharide loses Hydrogen(H) and another monosaccharide loses a Hydroxyl group(OH). It includes sucrose, lactose, and maltose. Sucrose(table sugar) is made of up glucose and fructose. Lactose(milk) is made up of glucose and galactose. While, maltose(found in grains) is made up of two parts glucose.
A polysaccharide means many. This includes starches or glucose polymers.
Carbohydrates mean part hydrogen2, carbon, and oxygen. (I think this is the one question on the quiz that I actually got right. Go me!)
Starch and cellulose are carbohydrates, macromolecules, polymers, and monomers. What's the difference between a polymer and monomer you may ask? A polymer is built from repeating units while monomers are built from links, like a chain continuously building.
Glycogen!! Most animals unknowingly store excess glucose by polymerizing it forming glycogen. The glycogen then breaks back down when the energy is needed.
Cellulose is common as being plant structural material. It's also common in wood, cotton, and paper. Hydrogen bonds are common in cellulose because there are many -OH groups and oxygen atoms in the "ring".
Now onto the experiment we did:
Benedict's:
We mixed different contents with Benedict's solution to find out if it was a monosaccharide or disaccharide. After mixing the contents with the solution, and letting it sit in heated water for about two minutes, we had our results. If the contents in the test tube turned a bright orange or orange-ish color, then the solution was a monosaccharide. If it turned a brown-ish color then the solution was a disaccharide, but if the solution had no change at all it was either water or a polysaccharide.
[a great online virtual lab is: http://bioweb.wku.edu/courses/Biol114/Online/Carbo/carbo1.asp]
Iodine:
We mixed a few different contents with iodine in a test tube. If the contents turned a bright, noticable blue color then the contents were startches. If they didn't then, well, they weren't starches.
[a great online virtual lab is: http://www.purchon.com/biology/food.htm]
Self Evaluation: I suck at taking tests/quizzes/whatever you want to call them. BUT!! It's a work in progress. I just need to find a way that I learn best, and I think I've found it!
A monosaccharide means one or single. It includes glucose, galactose, and fructose. Food examples of this includes honey, fruit, high-fructose corn syrup(which we get from fructose) and milk(from galactose).
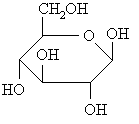 |
| Monosaccharide |
A disaccharide means two or double. When a disaccharide is created, one monosaccharide loses Hydrogen(H) and another monosaccharide loses a Hydroxyl group(OH). It includes sucrose, lactose, and maltose. Sucrose(table sugar) is made of up glucose and fructose. Lactose(milk) is made up of glucose and galactose. While, maltose(found in grains) is made up of two parts glucose.
 |
| Disaccharide |
A polysaccharide means many. This includes starches or glucose polymers.
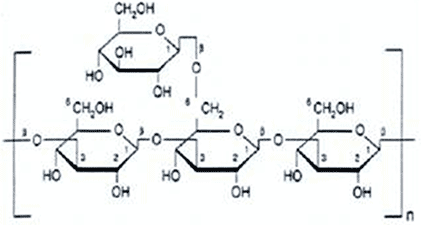 |
| polysaccharide |
Carbohydrates mean part hydrogen2, carbon, and oxygen. (I think this is the one question on the quiz that I actually got right. Go me!)
Starch and cellulose are carbohydrates, macromolecules, polymers, and monomers. What's the difference between a polymer and monomer you may ask? A polymer is built from repeating units while monomers are built from links, like a chain continuously building.
Glycogen!! Most animals unknowingly store excess glucose by polymerizing it forming glycogen. The glycogen then breaks back down when the energy is needed.
Cellulose is common as being plant structural material. It's also common in wood, cotton, and paper. Hydrogen bonds are common in cellulose because there are many -OH groups and oxygen atoms in the "ring".
Now onto the experiment we did:
Benedict's:
We mixed different contents with Benedict's solution to find out if it was a monosaccharide or disaccharide. After mixing the contents with the solution, and letting it sit in heated water for about two minutes, we had our results. If the contents in the test tube turned a bright orange or orange-ish color, then the solution was a monosaccharide. If it turned a brown-ish color then the solution was a disaccharide, but if the solution had no change at all it was either water or a polysaccharide.
[a great online virtual lab is: http://bioweb.wku.edu/courses/Biol114/Online/Carbo/carbo1.asp]
Iodine:
We mixed a few different contents with iodine in a test tube. If the contents turned a bright, noticable blue color then the contents were startches. If they didn't then, well, they weren't starches.
[a great online virtual lab is: http://www.purchon.com/biology/food.htm]
Self Evaluation: I suck at taking tests/quizzes/whatever you want to call them. BUT!! It's a work in progress. I just need to find a way that I learn best, and I think I've found it!
Monday, September 20, 2010
Article Summary
Cal-tech professor, James Heath and colleagues made a great discovery as if by mistake. "Almost all surfaces have a coating of water on them." he says. But this layer is hard to study due to the face that water is in constant flux. This "mistake" was made when Heath and colleagues were studying graphene on an atomically flat surface of mica. They found nanoscale 'island-shaped' structures trapped between the graphene and the mica. They conducted other experiments because they thought the 'islands' were water. The graphene sheet was atomically conformal(it hugged the water molecules as if it was shrink wrap). The first layer of water on the mica was two water molecules thick. The second layer was two water molecules thick and was like ice. The third(top) layer was just droplets of water. Heath and colleagues are now working on improving the resolution of the device so that it may be used to see the atomic structure of biomolecules like antibodies and other proteins.
Self Evaluation: I think this story is really interesting because it just shows that there are new discoveries in science almost everyday. This accident has opened a wide variety of windows for being able to see the atomic structure of very minuscule things.
Self Evaluation: I think this story is really interesting because it just shows that there are new discoveries in science almost everyday. This accident has opened a wide variety of windows for being able to see the atomic structure of very minuscule things.
Stomach Acid Lab
Self Evaluation: I enjoyed this activity, because we had some freedom of what antacids we could pour into the vinegar. Also, the reactions were interesting to watch. The results showed which antacids worked the best at raising the Ph Level. This lab taught me a lot about how the labels on antacids (and maybe other medicines) may lie to you about how effective they really are. Just because they say "maximum strength", or "extra strength" doesn't mean they actually work better.
Acids have excess H+ ions, making their Ph Level low, while bases have excess OH-, making their Ph Level higher. The lower the Ph Level the more acidic the substance is. Bases neutral out acids. So, since antacids are bases, they balance out the Ph Level of the acids. Water is neutral because the H+ and OH- balance out, making water completely neutral.
Tuesday, September 14, 2010
Properties of Water
Through experimentation and watching numerous videos about the properties of water, I have learned quite a bit. I broke down basically everything I've learned into key words, which I have conveyed through this powerpoint/movie.
Properties of Water
View more presentations or Upload your own.
If that doesn't work please refer to: http://www.slideboom.com/presentations/209499/Properties-of-Water
Sunday, September 5, 2010
Clinical Trial
Many children, ages 6 to 17, suffer from a disease called Attention Deficit-Hyper Disorder(ADHD). This disease causes kids (and later, adults) to be extremely hyper and not able to focus in a normal setting. Due to the increasing problems of this disease, many clinical trials have been attempted to try to find a drug that helps with the symptoms and affects of this disease. In this double blind trial, doctors tried the drug atomoxetine. A double blind trial is where neither the patient nor the doctor administering the drug know if the injection or capsule is atomoxetine or a placebo. Only a third party of scientists and doctors know which is which, but they never see the patient nor know of their name or other information. A placebo is known as a "sugar pill", because it is an inactive subtance that looks like medicine but contains absolutely no medicinal affects. This is used in clinical trials to see if the trial drug actually works or if the improvement is a mental state of mind.
Subscribe to:
Posts (Atom)









